CHEMICAL REACTION & EQUATION
By AMAN SINGH in 6 Sep 2023 | 05:25 pmWrite one equation each for decomposition reactions in which energy is supplied in the form of heat, light or electricity.
6 Sep 2023 | 05:25 pm
0
Likes
(a) Thermal decomposition reaction (Thermolysis)
Decomposition of potassium chlorate: When heated strongly, potassium chlorate decomposes into potassium chloride and oxygen. This reaction is used for the preparation of oxygen.
2KClO3 + Heat → 2KCl + 3O2
(b) Electrolytic decomposition reaction (Electrolysis)
Decomposition of sodium chloride: On passing electricity through molten sodium chloride, it decomposes into sodium and chlorine.
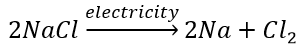
(c) Photodecomposition reaction (Photolysis)
Decomposition of hydrogen peroxide: In the presence of light, hydrogen peroxide decomposes into water and oxygen.
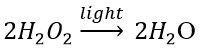
6 Sep 2023 | 05:26 pm
0
Likes
